 Image credit: RSC
Image credit: RSC摘要
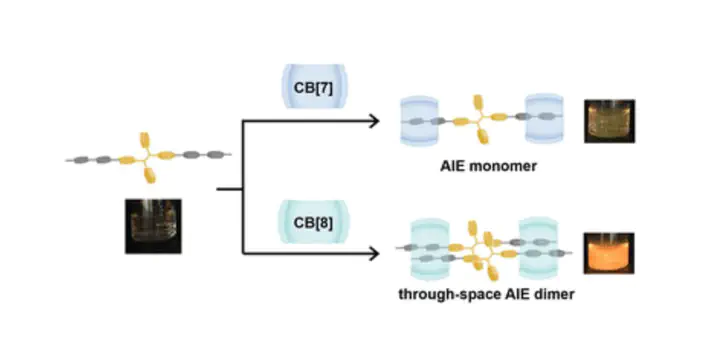
Aggregation-induced emission (AIE) of organic fluorophores is of broad interest for research in areas including chemistry, biology and materials science. Previous reports have mainly focused on enhancing fluorescence of various AIE entities including small molecules, biomacromolecules and nanoparticles. However, there remains a fundamental lack of understanding of the initial aggregation steps AIE cores undergo. Herein, we report the modification of an archetypal AIE fluorophore (tetraphenylethylene, TPE) with aryl pyridinium salts to form a TPE derivative. Employing cucurbit[n]uril macrocyclic hosts in the presence of this TPE derivative enables the controlled formation of discrete monomeric and dimeric structures, shedding light onto the early stages of aggregation. The discrete TPE dimer exhibits unprecedented photophysical behaviour with strong fluorescence in both the solid and liquid states. This supramolecular clamping strategy results in through-space dimerisation enhanced emission, providing detailed insights applicable for the design and formation of functional fluorescent (bio)materials.